To see the Blogging Hawks original post...
Wonders Lead to Discovery
Hello Blogging Hawks,
I know the previous class has tipped you off I am likely to visit your blog and, despite being very busy making DVDs/CDs and helping my local schools, I just had to drop in a comment about your latest post. I wonder if you all suspected I would interested in this post? I know previous classes learned of my interest in many things and geology as one in particular.
What a brilliant activity! I will have to try your experiment myself and see what results I can find.
The questions you started with are fascinating in themselves.
How are they formed? I know you discovered chemicals can invade spaces and, if the conditions are right, allow crystals to grow. what a natural wonder!
How long does it take? I think you also realised how long depends on the conditions. They can take millions of years or you can make them in a day. Did you notice no two of your geodes were exactly the same?
Where do you find them? Over the years, I have come across crystals protruding from the ground and clusters on rocks. It seems a game of chance if you're in a spot known for crystals or geodes. When we do a search on the internet, we realise geodes can be found in many places around the world. Here is a Wikipedia link on geodes...
Geodes
Can you really dye them? When I was your age, I wondered the same question. Like in your picture, I had seen beautiful colours in geode slices but soon learned they weren't always natural.
Crystals can, of course, be naturally coloured. We know diamonds can be clear, blue, yellow, brown, green, purple orange or even pink depending on small defects or impurities*. Quartz can also be clear, purple (amethyst), yellow (citrine) and other colours. I don't have any dyed geodes in my collection as I prefer natural colouring.
*Here's a little information I found. Blue diamonds are blue because they have boron impurities. Boron is part of borax.
Below you can see photos of some crystals in my collection.
Quartz from Northern Territory, Australia
Smoky Quartz
Citrine Quartz from Brazil
Iron Pyrite (Fool's Gold) from Australia's Northern Territory
Mr. B is certainly fabulous to bring in such an interesting experiment. I also liked the option to create a crystal snowflake. They may not look like a geode but each is special as, just like snowflakes and us, no two are exactly alike. That's what has interested me about geodes, each is unique even if they look almost exactly the same.
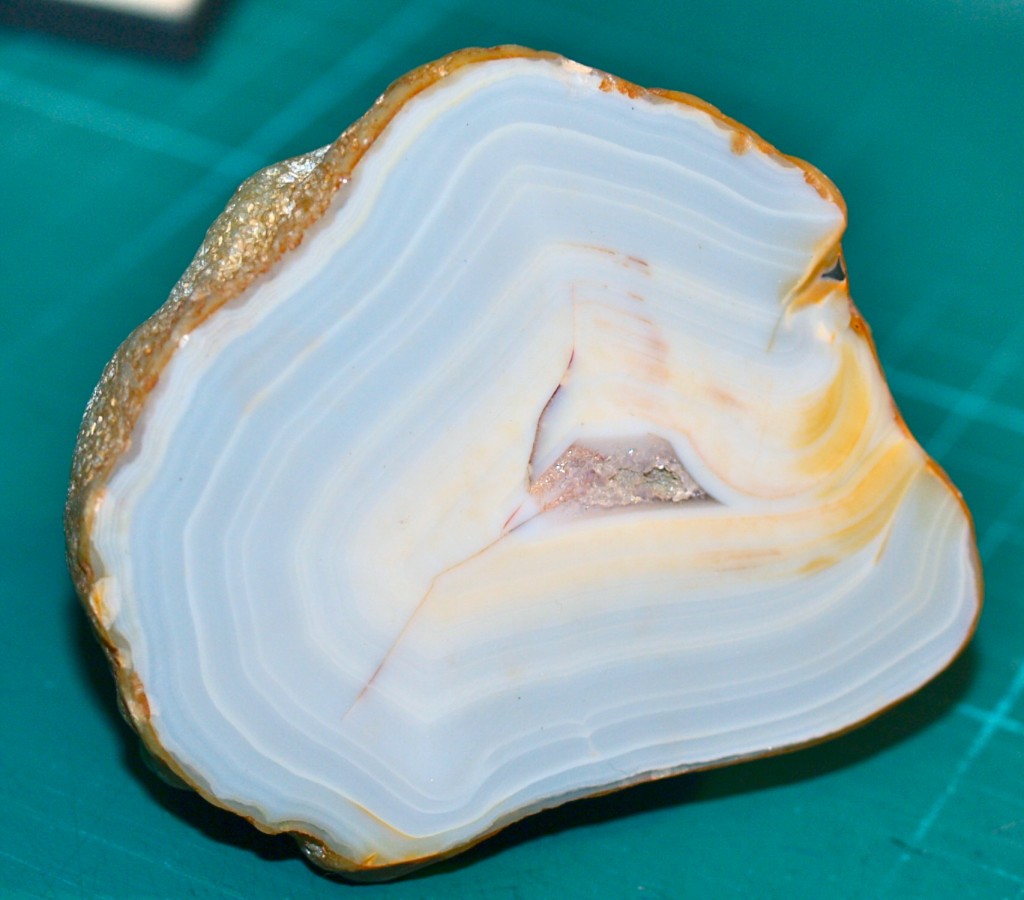
Look at the pictures below. Each is of a Brazilian geode in my collection. One has been cut in half while the other is complete. I decided long ago never to cut open the complete geode so what's inside remains a mystery. It could be incredibly beautiful but I treasure the intact geode. I wonder if any of you could resist cutting it open?
Looking at your reflections...
Adam - You have shown what science experiments are often about. We take steps and wait to see
the results.
Shaye - I like your simile, "looks like a tiny city the way it sparkles". Good use of descriptive writing makes writing stories or explaining science easier to understand for readers and interesting.
Faith - You packed quite a bit of information into your comment. Adding "in my opinion" is a very good phrase. It tells readers the idea is yours and suggests others might have other ideas. You also introduced an idea, i.e. removing the yolks and albumin (white) from eggs to make them hollow. Did you know there are collections of bird eggs often found in museums, universities and even private collections? Collectors normally make small holes at either ends of an egg and blow out the contents. Can you imagine doing this for an ostrich egg?
Haya - Aquamarine may not be my birthstone but I do like the idea of making blue crystals. I like your suggestion it might be possible to make your name in crystals. I know it will work because you only need something for the crystals to grow on. Raindrops and snowflakes are the same, they need something to form on. In the sky, it might simply be dust.
On checking, it seems my birthstone is either topaz or citrine. I have citrine quartz in my collection. Here is a close-up photo of citrine quartz crystals
Marcus - You have shown your knowledge of stones. Obsidian, volcanic glass, can be very dark and forms when lava, high in silica, cools quickly so minimal crystals form. I have seen natural obsidian in the crater of a volcano in New Zealand and have some in my collection (bought, not taken from the volcano as it isn't permitted). Below is a photo I took about 40 years ago on the edge of a crater in Mt. Tarawera, New Zealand.
-
Like you, I also like the reflection of light on crystal surfaces. What else is borax used for? Here is a Wikipedia link.
Borax
Liam - How interesting. You used green dye but your crystals seemed blackish. How could this be? Sometimes dyes can concentrate (get stronger) as the mix dries out or perhaps the crystals on your star were growing on something dark. I love mysteries. They encourage us to suppose what might have happened. Some of our great discoveries have come about when experiments didn't turn out as expected.
Riley - You have given a very good explanation of the process you used to make crystals. I also liked your suggestion the crystals looked life-like. Crystals grow as do we so it seems a little like life. Imagine if we were able to use time-lapse photography (pictures taken at regular times apart) to photograph the growth of your crystals then showed the photos one after the other as in a movie. We would see the crystals forming and growing.
Alvin - I liked your "wonder". The chemical reaction works quicker when in hot water. You can see this with sugar dissolving in water. If two of you each placed a spoon of sugar in containers of water where one had hot water and the other cold, I suspect you would find the sugar is dissolved more quickly in the hot water. What do you think?
Anita - I liked your use of "Step 1" and "Step 2" in your comment. Science, particularly chemistry, uses steps to carry out experiments. It looks like a cooking recipe. Follow the steps and you should bake a cake. Follow your class experiment and you should get crystals.
Why use borax? Borax dissolves easily in water and has water in its formula. Let's look at the chemistry involved...
Borax is sodium tetraborate decahydrate but I think it's easy to remember borax. The longer name tells us the chemical contains the elements sodium, four parts boron, and oxygen as well as 10 parts water. It's written like this...
Na is sodium, B is boron and tetra tells us 4 parts, O is oxygen and there is also 10 parts water. It is a type of salt and can be found in crystal form. Below is a public domain photo of borax crystal I found through Wikimedia Commons. You can see borax can form crystals.

This work has been released into the public domain by its author, Aramgutang at the English Wikipedia project.
Carter - I like your explanation of the steps needed in order to carry out your class geode experiment. One of the keys to scientific research is recording information. Experimenters have to not only record what happens in an experiment, they have to record how an experiment was done. This is so other scientists can repeat an experiment to check the results.
Marah - I like your description of the appearance of your crystals. My imagination was sparked by the thought of a million cazillion crystals sparkling in the light. I know I enjoy the sparkle as light bounces off the facets (faces) of the crystals. I also liked your thought on what might happen if the borax wasn't completely dissolved. Science is full of "what if" questions and experiments to discover the answers.
Colby - How did the borax mix in with the dye? How and why do crystals grow on a pipe cleaner? What a great questions. It's good to be able to follow experiments but to wonder why things happen is real scientific thinking. Questioning why then finding answers is the sign of a mind full of curiosity.
William - I like your "wonder" thinking when you wondered if the crystals would continue to grow if you returned your geode to the mixture. I suspect, providing the chemicals aren't all used, the crystals would continue growing. Imagine filling and egg and making a solid crystal geode. Your idea made me wonder what might happen if, after the crystals have grown in the first borax/dye mix, the eggs were placed in a different dye colour/borax mix? Would you get two coloured crystals? Would the new mix dissolve the old crystals? Would the first crystals change colour? These questions could be a completely new experiment.
Sofie - Because of the photo, I can see your crystal star so I agree it is really cool. I also read your birthstone is sapphire. The area of Australia I live in is known as the Sapphire Coast. It's known for the colours of the sea and sky in summer. Below is a photo taken from a trail in my town.
- Schools and students have permission to use this graphic for non-commercial, educational purposes.
Did you know there is something known as the Moh Scale? It's used to describe how hard minerals are. At the very top at level 10 hardness is the diamond. Sapphire comes in at 9 so it is amongst the hardest.
MOH SCALE
Robert - It can happen in experiments. What was expected to happen doesn't exactly work out as planned but this has led to some good results. Perhaps you have used post-it sticky notes? They work so well because they can be stuck on, removed and replaced. Did you know the glue used was an unexpected result of an experiment? A scientist was trying to develop a very strong glue. The results of one experiment was the glue now used on post-it notes. He realised it was sticky enough to hold but could be removed and reused. I find science fascinating, especially when it finds something unexpectedly good.
Olivia - A couple people have written about their fake geodes. I find the "fake" idea interesting. If we were to accidentally spill chemicals on the ground and they seeped through the soil, found a space, and started to grow as crystals, would they be fake or real? People didn't try to make them but they could happen. Real geodes are ones made in nature but your geodes were made in class. Real or fake, aren't they amazing?
Thomas - I wonder if geodes can change? I liked your question. You now know natural geodes take much longer to form than the ones you made in class. Forming crystals can take a great deal of time and need the right conditions. Think of this unusual crystal idea. Diamonds can only be formed when deep underground under pressure and heat for a very long time. They are made of carbon. Can they change? Did you know they tend to only come near the surface when brought up by volcanic eruption? Being made of carbon, do you think some would burn up in the magma? Imagine burning diamonds.
This is not a diamond. It's a glass replica. I can't afford to add a real diamond to my collection. 🙂
Thomas's thought may have been correct. If someone accidentally bumped the borax/dye mix when crystals started forming, the crystals might not form correctly. Also, using too little borax might mean crystals don't form correctly because there isn't enough of the chemicals. I like mysteries in science. It can be fun trying to find the answers.
Mani - Now there's an interesting idea, would the process work with laundry powder?
I looked up information about borates in laundry powder and found they have a number of effects to help in the cleaning process but no information about using borate detergent to make crystals. I think it might depend on how much borax was in the detergent. There might not be enough to have good crystal growth or possibly even the detergent might stop growth. That would need another experiment to find answers.
Saadia - It can be cool to experiment but I think the coolest part would be seeing how all of the geodes and stars looked at the end. Imagine having many crystal stars hanging in the sunlight. Light would be reflecting off them in so many directions.
Luisa - I also think your parents would be proud of what you have made. I know I would want to try the experiment again but remember to always have adult help when working with chemicals. When I have some time, I'm going to have to try to make geodes and stars just like your class. I wonder how they might look?
Prayers - I'm glad your class mentioned making either geodes or stars. I might have though of geodes but not stars. I wonder what other shapes I could make? Would I be able to join the shapes to make crystal patterns?
I also liked your question as to whether you could make crystals without the borax. You can also try using salt or sugar. Dissolve salt or sugar in warm water and allow the water to evaporate off in the sun. Small crystals would form but I think you would find your borax crystals are larger.
Aleah - Your curiosity asked you questions I also found interesting. Would the eggshell inside be white or would the dye have changed the shell to colour? I suspect there might have been some change of colour but that's a guess. I would also guess they feel like real crystals because they are real crystals you just happened to grow.
Bryan - I wonder how the borax turns to crystals? What a great "wonder". Chemistry, the way chemicals can work together, is very interesting. The borax dissolves easily in water and then crystalises out again. How?
HOW CRYSTALS FORM
Without dye, the borax would form white crystals. Look at the picture posted for Anita and you will see what natural borax crystals look like.
Oliver - I like your description of the snowflakes being uncanny. Uncanny means 'unnaturally strange 'but we know crystals form naturally. I also find them uncanny because their sometimes beauty seems unnatural. Examining crystals and other stones under a magnifying glass or microscope can unlock some amazing images. I don't have a microscope but I did take some close up photos of some of my small crystals in one of my geodes. The first photo shows the geode and the other three are close up photos.
There are so many wonders in our world and so many I have yet to discover. All we need do is keep our minds and eyes wide open to the possibilities and our curiosity keen to know answers. Every day can be a learning experience just as reading your post and preparing this extended comment has been for me.